- APPLY FOR SLOT
- Internal Users
- External Users
- SLOT BOOKING STATUS
Circular Dichroism Spectropolarimetry Laboratory

Phone : +91-3222-283300
Location : OB / FF / 10, CRF
Facilitator :
Dr. Agneyo Ganguly, Biotechnology
Email: agneyo@iitkgp.ac.in, Contact:+91-3222-282896
For Internal Users - Click Here to apply for Slot
For External Users - Click Here to apply for Slot
Objectives
CD Spectroscopy measures differences in the absorption of left-handed polarized
light versus right-hand polarised light which arises due to structural asymmentry
of an optically active substance.
It's a powerfull tool to analyze the structure of biomolecules and their interaction
with various ligands.
Changes in the CD spectra reflect a perturbation in the structure of biomolecules
broughht about by changes in conditions like temparatur,pH or drug
binding.Protein folding/unfolding can be followed by changes in the CD spectra.
Besides chirality of small molecules can be determined with the help of CD
spectroscopy.
Stereochemistry of producys through enzymatic reactions is also an important
activity of CD spectroscopy,which is related to the 3D-structure of the active site
of en enzyme
People

Dr. Agneyo Ganguly
Facilitator
Biotechnology
agneyo@iitkgp.ac.in
+91-3222-282896

Mr. Sudhir Kr. Moharana
Technical Staff
Central Research Facility
sudhirkm@hijli.iitkgp.ac.in
+91-3222-269932
Equipment Details
Circular Dichroism Spectropolarimeter
Model: JASCO (J-810)
Specification of the Instrument: CD Measurements are being carried out using 150W air-cooled xenon lamp as source of light with measurement wavelength range from 163 to 900nm (standard detector).It provides a CD resolution of 0.0005 mdeg,0.01 mdeg,0.1 mdeg (at ± 10mdeg,± 200mdeg,± 2000mdeg full scale respectively). Protein secondary structure can be determined by CD spectroscopy in the far-UV spectral region (190-240nm).For estimation of protein secondary structure the reference CD spectra published in "Table III of Methods in Enzymology,130,208-269 (1986) by Prof. Jen Tsi Yang.Biochemistry/Biophysics Dept.University of California,San Francisco,USA" are used.The data contained in this table is used as a reference file.
Manufacturer details: JASCO International Company Limited(Hachioji,Tokyo, JAPAN)
Date and cost of Procurement: 16th Aug 2002; Yen 74,25,000.00 ~ Rs 30,03,190.00/-
Purchase source (funding): Institute
Year of installation: 16th Feb.2003
Utility and Working Principal
Circular Dichroism (CD) is observed when optically active matter absorbs left and right hand circular polarized light slightly differently. It is measured with a CD spectropolarimeter. The instrument needs to be able to measure accurately in the far UV at wavelengths down to 190-170 nm. The difference in left and right handed absorbance A(l)-A(r) is very small (usually in the range of 0.0001) corresponding to an ellipticity of a few 1/100th of a degree. The CD is a function of wavelength. CD spectroscopy has become a powerful tool to analyse the structure of biomolecules and their interaction with various ligands. Changes in the CD spectra reflect a perturbation in the structure of biomolecules brought about by changes in conditions like temperature, pH or drug binding. Protein folding/unfolding can be followed by changes in the CD spectra. For distinct types of secondary structure present in peptides, proteins and nucleic acids, CD spectra are different. The analysis of CD spectra can therefore yield valuable information about secondary structure of biological macromolecules.
Sample Details
The concentration at which CD is measured is sample dependent. It is necessary to consider the proper range of OD (usually OD of ~ 1). If the OD reaches approx. 2, noises increase and the spectrum may be distorted due to luminescence, depending on the sample. Although CD is not much affected by the turbidity of the solution, a short cell should be used if the photomultiplier voltage exceeds 400 V. Small optical path length of 5 mm or less are mainly used to expand the short-wavelength limit of the far-UV region, they are also used to decrease the sample OD. The cells with a large optical path length of 2 cm or more are used when the sample concentration is smaller. However, a high tension voltage (HT) of the photomultiplier detector of 500 V or more is not recommended because noise increases with the exponential function of the OD when the HT increased. Apart from the above, sufficient amount of the buffer used for sample preparation is needed for baseline correction and rinsing the cells.
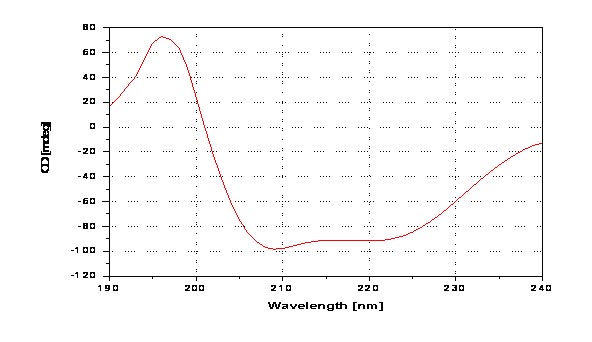
Sample Title
CD spectra of 10µmol/L Bovine Serum Albumin (BSA) in Distilled Water using quartz cell with 0.1cm cell path length at room temperature.
|
HELIX |
24.4 % |
|
BETA |
25.0 % |
|
TURN |
21.5 % |
|
RANDOM |
29.1 % |
Secondary Structure Estimation Report of Bovine Serum Albumin (BSA)
